Back to Articles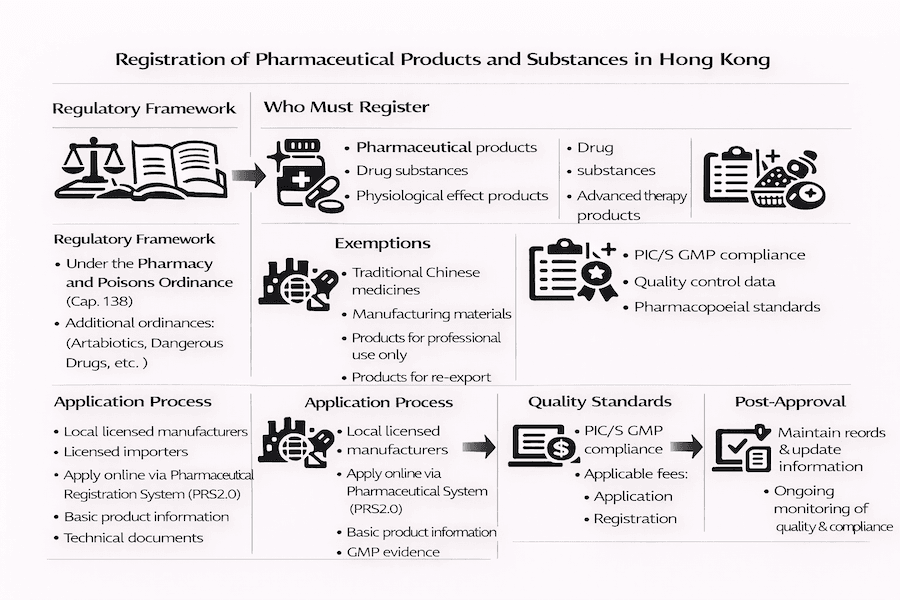
Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
Registration
July 20, 2024
Approximately 5 minutes
Professional Guide: Registration of Pharmaceutical Products and Substances in Hong Kong

Professional Guide: Registration of Pharmaceutical Products and Substances in Hong Kong
This guide provides essential information for healthcare professionals, manufacturers, and distributors regarding the registration of pharmaceutical products and substances in Hong Kong.
Regulatory Framework
Governing Legislation
-
Pharmacy and Poisons Ordinance (Cap. 138)
- Primary legislation for pharmaceutical products
- Registration requirements and procedures
- Enforcement provisions
-
Supporting Ordinances
- Antibiotics Ordinance (Cap. 137)
- Dangerous Drugs Ordinance (Cap. 134)
- Undesirable Medical Advertisements Ordinance (Cap. 231)
Registration Requirements
Products Requiring Registration
-
Pharmaceutical Products
- Products with medicinal claims
- Products containing drug substances
- Products affecting physiological functions
- Advanced therapy products
-
Exemptions
- Traditional Chinese medicines
- Manufacturing materials
- Professional use products
- Re-export products
- Clinical trial products
Application Process
Eligibility Criteria
-
Local Manufacturers
- Must be a licensed manufacturer
- Must have GMP certification
- Must comply with PIC/S standards
-
Importers
- Must be a licensed wholesale dealer
- Must have manufacturer authorization
- Must meet import requirements
Required Documentation
-
Basic Information
- Business registration certificate
- Manufacturer's authorization
- Application authorization
-
Technical Documentation
- GMP certificate
- Free Sale Certificate
- Product specifications
- Quality control data
-
Special Requirements
- Animal origin materials documentation
- Multiple manufacturer declarations
- Bioequivalence data (if applicable)
Quality Standards
Manufacturing Requirements
-
GMP Compliance
- PIC/S GMP standards
- Quality management system
- Facility requirements
-
Quality Control
- Release specifications
- Stability data
- Validation protocols
Pharmacopoeial Standards
- Chinese Pharmacopoeia
- British Pharmacopoeia
- European Pharmacopoeia
- International Pharmacopoeia
- Japanese Pharmacopoeia
- United States Pharmacopoeia
Application Submission
Online System
- Use PRS 2.0 system
- Submit electronic documents
- Pay application fee (HK$1,100)
Supporting Documents
- Submit original certificates
- Provide certified copies
- Include translations if needed
Post-Approval Requirements
Registration Fee
- HK$1,370 per product
- Payable upon approval
- Required for certificate collection
Compliance Obligations
-
Documentation
- Maintain records
- Update information
- Report changes
-
Quality Assurance
- Regular testing
- Stability monitoring
- Complaint handling
Special Considerations
Patent Rights
- Board does not consider patents
- Applicants responsible for compliance
- Potential infringement risks
- Legal consultation recommended
Multiple Manufacturers
- Single manufacturing pathway preferred
- Alternative pathways require separate applications
- Clear documentation of roles required
Application Tracking
Progress Monitoring
- Track application status
- Quote file reference
- Contact Drug Registration Unit
- Follow up as needed
Important Notes
Legal Compliance
- Follow current legislation
- Monitor regulatory updates
- Maintain documentation
- Ensure quality standards
Professional Responsibility
- Verify product eligibility
- Ensure complete documentation
- Monitor application progress
- Maintain compliance
Contact Information
Drug Office, Department of Health
- Website: www.drugoffice.gov.hk
Additional Resources
- Legislation: www.elegislation.gov.hk
Ask Anything
We'll follow up with you personally.
100% response rate • Reply within 7 business days
Important Disclaimer