Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 5, 2024
Approximately 5 minutes
Traditional Chinese Medicine (TCM) Regulations in Hong Kong
Traditional Chinese Medicine (TCM) Regulations in Hong Kong
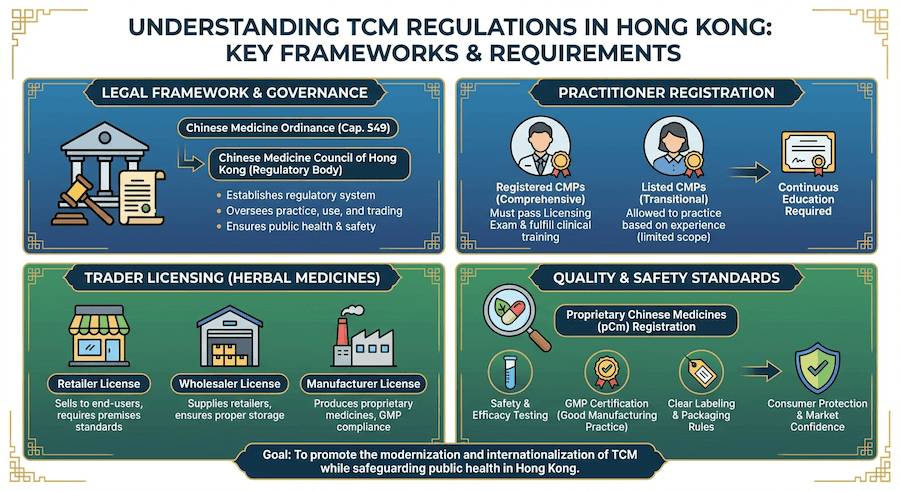
Traditional Chinese Medicine has a long history in Hong Kong and is regulated under a comprehensive legal framework. The Chinese Medicine Ordinance provides the regulatory structure for TCM practitioners, Chinese medicines, and related activities.
Regulatory Framework
The Chinese Medicine Council of Hong Kong oversees the regulation of TCM under the Chinese Medicine Ordinance (Cap. 549). This includes registration of practitioners, regulation of Chinese medicines, and licensing of trading activities.
Key Regulatory Bodies:
- Chinese Medicine Council of Hong Kong
- Chinese Medicine Board
- Chinese Medicines Board
- Department of Health (Chinese Medicine Division)
TCM Practitioner Registration
Registration Categories:
- Registered Chinese Medicine Practitioners (CMP)
- Listed Chinese Medicine Practitioners
- Registered Chinese Medicine Practitioners (Bone-setting)
- Registered Chinese Medicine Practitioners (Acupuncture)
Registration Requirements:
- Recognized qualifications
- Clinical experience
- Examination requirements
- Continuing education
- Professional conduct standards
Chinese Medicine Regulation
Classification of Chinese Medicines:
- Proprietary Chinese Medicines (pCm)
- Chinese Herbal Medicines
- Chinese Medicine Materials
Registration Process:
- Product registration application
- Safety and efficacy assessment
- Quality standards compliance
- Labeling requirements
- Manufacturing standards
Proprietary Chinese Medicines (pCm)
Registration Requirements:
- Product information and composition
- Manufacturing details
- Safety data
- Efficacy evidence
- Quality control standards
Categories:
- Category I: Established formulations
- Category II: Modified traditional formulations
- Category III: New formulations
Documentation:
- Product registration form
- Manufacturing authorization
- Quality standards
- Clinical data (if required)
- Labeling information
Chinese Herbal Medicines
Import and Trading:
- Wholesale dealer license
- Import license requirements
- Storage and handling standards
- Record keeping obligations
- Quality assurance measures
Quality Standards:
- Identification requirements
- Purity standards
- Contamination limits
- Pesticide residue limits
- Heavy metal limits
Manufacturing Requirements
Good Manufacturing Practice (GMP):
- Facility design and construction
- Equipment and utilities
- Personnel and training
- Production controls
- Quality control systems
Manufacturing License:
- Application requirements
- Facility inspection
- Compliance assessment
- License conditions
- Renewal procedures
Trading and Distribution
Wholesale Dealer License:
- Business registration
- Premises requirements
- Personnel qualifications
- Record keeping systems
- Storage conditions
Retail Requirements:
- Listed Seller of Chinese Medicines
- Premises standards
- Display requirements
- Sales restrictions
- Record keeping
Quality Control and Testing
Testing Requirements:
- Identity testing
- Purity analysis
- Microbiological testing
- Heavy metal analysis
- Pesticide residue testing
Laboratory Standards:
- Accredited testing facilities
- Validated methods
- Reference standards
- Quality assurance
- Documentation requirements
Labeling and Advertising
Labeling Requirements:
- Product name and composition
- Manufacturer information
- Batch number and expiry date
- Storage instructions
- Usage directions
Advertising Restrictions:
- Prohibited claims
- Therapeutic claims
- Approval requirements
- Penalty provisions
- Compliance monitoring
Import and Export
Import Requirements:
- Import license application
- Certificate of registration
- Customs clearance
- Documentation requirements
- Country of origin
- CITES import licences (if applicable)
Export Requirements:
- Export license
- Certificate of origin
- CITES export licences (if applicable)
- Destination country requirements
- Documentation compliance
Enforcement and Penalties
Inspection Powers:
- Premises inspection
- Sample collection
- Document examination
- Investigation authority
- Enforcement actions
Penalties:
- Administrative sanctions
- Criminal prosecution
- License suspension
- Product recall
- Financial penalties
International Harmonization
WHO Guidelines:
- Quality standards
- Safety requirements
- Good practices
- Regulatory harmonization
- International cooperation
Regional Cooperation:
- ASEAN harmonization
- Mutual recognition
- Information sharing
- Joint inspections
- Capacity building
Challenges and Opportunities
Common Challenges:
- Quality standardization
- Safety assessment
- Regulatory compliance
- International trade
- Consumer protection
Market Opportunities:
- Growing global demand
- Innovation in formulations
- Technology integration
- Export potential
- Healthcare integration
Technology and Innovation
Modern Approaches:
- Analytical technologies
- Quality control automation
- Digital documentation
- Traceability systems
- Data management
Research and Development:
- Clinical studies
- Pharmacological research
- Quality improvement
- New product development
- Evidence-based medicine
Compliance Best Practices
Documentation Management:
- Comprehensive records
- Version control
- Regular updates
- Audit trails
- Backup systems
Quality Assurance:
- Supplier qualification
- Incoming inspection
- Process controls
- Final testing
- Continuous monitoring
Staff Training:
- Regulatory awareness
- Technical competency
- Quality procedures
- Safety protocols
- Continuing education
Future Developments
Regulatory Evolution:
- Risk-based approaches
- International harmonization
- Digital transformation
- Evidence requirements
- Safety monitoring
Industry Trends:
- Modernization initiatives
- Quality improvements
- Global expansion
- Innovation support
- Integration with conventional medicine
Conclusion
TCM regulation in Hong Kong provides a comprehensive framework for ensuring quality, safety, and efficacy. Success requires understanding regulatory requirements, implementing robust quality systems, and maintaining compliance with evolving standards.
For expert guidance on TCM regulatory compliance, contact ElendiLabs for specialized consultation.
Ask Anything
We'll follow up with you personally.