Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
October 15, 2024
Approximately 5 minutes
Setting Up Your Pharmaceutical Business in Hong Kong
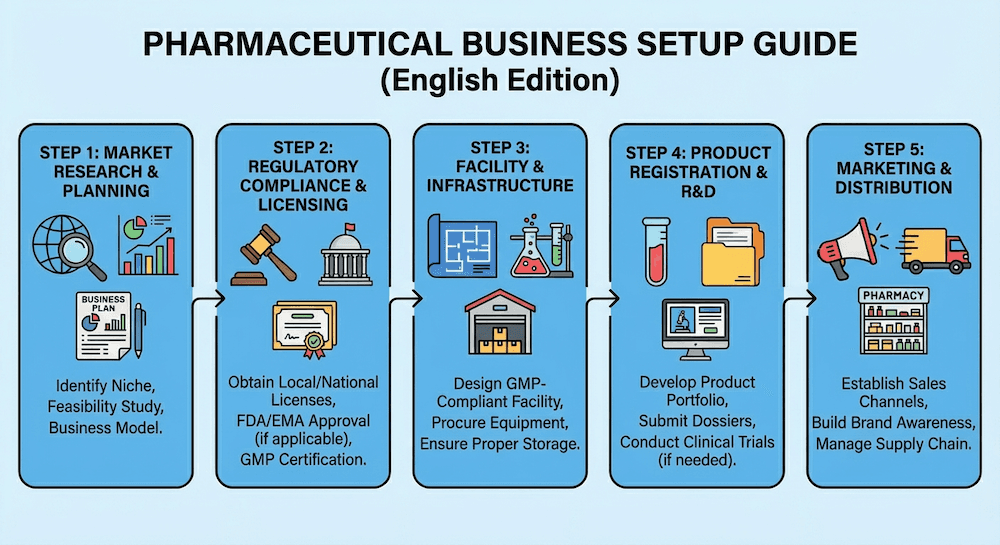
Setting Up Your Pharmaceutical Business in Hong Kong
Establishing a pharmaceutical business in Hong Kong requires careful planning, regulatory compliance, and strategic decision-making. This comprehensive guide covers all aspects of pharmaceutical business setup.
Business Planning and Structure
Business Entity Types
Choose the appropriate business structure:
- Limited Company (most common for pharmaceutical businesses)
- Sole Proprietorship
- Partnership
- Branch Office of overseas company
Key Considerations:
- Liability protection
- Tax implications
- Regulatory requirements
- Investment and funding needs
- Future expansion plans
Regulatory Licensing Requirements
Core Licenses for Pharmaceutical Business
Wholesale Dealer License (WDL)
Required for:
- Wholesale distribution of pharmaceutical products
- Import and export activities
- Storage and handling of medicines
- Supply to licensed premises
Manufacturing License
Required for:
- Local pharmaceutical manufacturing
- Good Manufacturing Practice (GMP) compliance
- Quality control laboratories
- Packaging and labeling operations
Retail Licenses
- Authorized Seller of Poisons (ASP) for full pharmacy services
- Listed Seller of Poisons (LSP) for over-the-counter medicines
Specialized Licenses
- Dangerous Drug License (for controlled substances)
- Psychotropic Substances Permit
- Antibiotics Permit (for antibiotic products)
- Clinical Trial Certificate (for research activities)
Premises and Facility Requirements
Wholesale and Storage Facilities
Requirements include:
- Adequate storage space and capacity
- Temperature and humidity control systems
- Security measures and access controls
- Segregation areas for different product types
- Quality control and quarantine areas
Good Distribution Practice (GDP) Compliance
- Written procedures and documentation
- Staff training and competency
- Temperature monitoring and validation
- Traceability and recall procedures
- Regular audits and reviews
Manufacturing Facilities (if applicable)
- Good Manufacturing Practice (GMP) compliance
- Validated manufacturing processes
- Quality control laboratories
- Environmental monitoring
- Waste management systems
Staffing and Personnel
Key Personnel Requirements
- Responsible Person (RP) with appropriate qualifications
- Registered Pharmacist (for ASP operations)
- Quality Assurance personnel
- Regulatory affairs specialists
Qualifications and Training
- Relevant pharmaceutical education and experience
- Hong Kong registration and licensing
- Ongoing professional development
- Compliance training and competency assessment
Quality Management Systems
ISO Standards Implementation
Consider implementing:
- ISO 9001 (Quality Management)
- ISO 13485 (Medical Devices Quality Management)
- ISO 14001 (Environmental Management)
- ISO 45001 (Occupational Health and Safety)
Documentation Requirements
- Quality manual and procedures
- Standard Operating Procedures (SOPs)
- Batch records and documentation
- Training records and competency assessments
- Audit reports and corrective actions
Regulatory Compliance Framework
Pharmacovigilance Systems
Establish systems for:
- Adverse event monitoring and reporting
- Signal detection and evaluation
- Risk assessment and management
- Safety database maintenance
Product Registration and Lifecycle Management
- Initial product registration
- Variation applications
- Renewal procedures
- Post-market surveillance
Import/Export Compliance
- License applications and renewals
- Documentation requirements
- Customs procedures
- International harmonization
Financial Considerations
Startup Costs
Budget for:
- Business registration and licensing fees
- Premises setup and equipment
- Staff recruitment and training
- Professional services and consultancy
- Working capital and inventory
Ongoing Operational Costs
- License renewal fees
- Facility maintenance and utilities
- Staff salaries and benefits
- Professional indemnity insurance
- Regulatory compliance and auditing
Funding Options
- Personal investment
- Bank loans and financing
- Venture capital and private equity
- Government grants and incentives
- Strategic partnerships
Technology and IT Infrastructure
Essential Systems
- Inventory management systems
- Customer relationship management (CRM)
- Enterprise resource planning (ERP)
- Document management systems
- Compliance tracking systems
Data Protection and Security
- Personal data privacy compliance
- Cybersecurity measures
- Backup and disaster recovery
- Access controls and audit trails
Market Entry Strategies
Target Market Analysis
- Healthcare institutions and hospitals
- Retail pharmacies and clinics
- Government procurement
- Export markets and international distribution
Partnership Opportunities
- Local distributors and agents
- Healthcare providers
- Research institutions
- International pharmaceutical companies
Common Challenges and Solutions
Regulatory Complexity
- Challenge: Navigating complex licensing requirements
- Solution: Professional regulatory consulting
Competition and Market Access
- Challenge: Established market players
- Solution: Differentiation and niche positioning
Talent Acquisition
- Challenge: Skilled pharmaceutical professionals
- Solution: Competitive packages and development programs
Technology Implementation
- Challenge: System integration and validation
- Solution: Phased implementation and expert support
Best Practices for Success
1. Regulatory Excellence
- Stay updated with regulatory changes
- Invest in compliance systems and training
- Maintain strong relationships with authorities
- Prepare for inspections and audits
2. Quality Focus
- Implement robust quality management systems
- Regular internal audits and reviews
- Continuous improvement culture
- Customer satisfaction monitoring
3. Strategic Planning
- Clear business objectives and milestones
- Regular strategy reviews and updates
- Risk assessment and mitigation
- Succession planning and continuity
4. Professional Networks
- Industry associations and chambers
- Professional development groups
- Regulatory forums and conferences
- International partnerships
Future Opportunities
Digital Health and Innovation
- Telemedicine and digital therapeutics
- Artificial intelligence and machine learning
- Blockchain and supply chain transparency
- Personalized medicine and genomics
Regional Expansion
- Greater Bay Area integration
- ASEAN market opportunities
- Belt and Road Initiative participation
- Cross-border e-commerce
Conclusion
Setting up a pharmaceutical business in Hong Kong requires comprehensive planning, regulatory expertise, and strategic execution. Success depends on understanding local requirements, building strong operational capabilities, and maintaining compliance excellence.
For expert assistance in pharmaceutical business setup, contact ElendiLabs for comprehensive consultation and support services.
Ask Anything
We'll follow up with you personally.
Related Articles
Approximately 5 minutes
Pharmacy vs Medicine Store: Understanding the Key Differences
Learn the crucial differences between pharmacies and medicine stores in Hong Kong, including licensing requirements, services offered, and regulatory distinctions.
Approximately 5 minutes
ASP vs LSP License: Which One Do You Need?
Learn the differences between Authorized Seller of Poisons (ASP) and Listed Seller of Poisons (LSP) licenses and determine which is right for your business.
Approximately 5 minutes
Professional Guide: Registration of Pharmaceutical Products and Substances in Hong Kong
A comprehensive guide for healthcare professionals and industry stakeholders on the registration requirements, procedures, and compliance for pharmaceutical products and substances in Hong Kong.