Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 15, 2025
Approximately 5 minutes
Listing Procedures for Medical Device Distributors in Hong Kong: A Guide to GN-09
Listing as a Medical Device Distributor in Hong Kong: Our Insights from GN-09
From what we've seen, getting medical devices safely from manufacturers to patients in Hong Kong involves many key players. The Medical Device Administrative Control System (MDACS), overseen by the Department of Health's Medical Device Division (MDD), is all about making sure these devices are safe, high-quality, and perform well throughout their entire journey. For companies that distribute medical devices in Hong Kong, the "Guidance Notes for Listing of Distributors of Medical Devices" (GN-09) lays out the specific steps for them to voluntarily list their operations. While it's a voluntary choice, according to our experience, being listed really shows your commitment to good practices and strengthens confidence in the entire medical device supply chain in Hong Kong.
What is the definition of a medical device in Hong Kong?
When we talk about "medical devices" in Hong Kong, what exactly does the Department of Health's Medical Device Division (MDD) consider to be one? According to their guidelines, a medical device is essentially any instrument, apparatus, machine, appliance, implant, software, material, or similar item. It's important that the manufacturer intends it to be used, either alone or with other things, for specific medical purposes in humans.
These purposes include:
- Diagnosing, preventing, monitoring, treating, or easing diseases.
- Diagnosing, monitoring, treating, easing, or compensating for an injury.
- Investigating, replacing, modifying, or supporting the body's structure or how it works.
- Supporting or sustaining life.
- Controlling conception (like contraception).
- Disinfecting other medical devices.
- Providing medical information by examining samples from the human body (this is what IVDMs do!).
Crucially, a medical device does not achieve its main effect in or on the body through drugs, immune responses, or metabolism. However, it might be helped in its function by such means. So, it's pretty broad, covering everything from a simple bandage to complex diagnostic software!
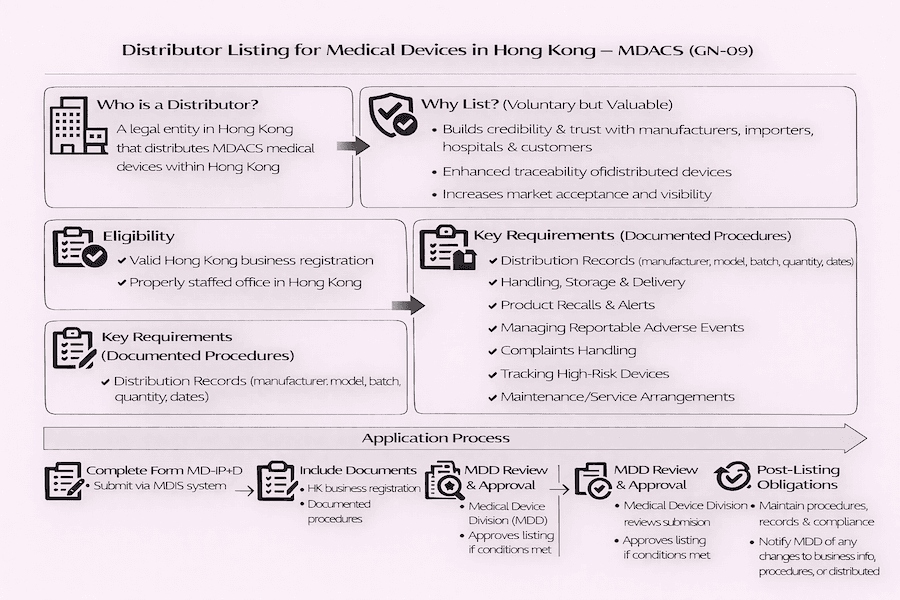
Who is a Medical Device Distributor in Hong Kong?
Under the MDACS, a "Distributor" is generally described as a company (a "legal person") that hands out or supplies any medical devices that fall under the MDACS rules within Hong Kong. From our understanding, this usually means they bring devices into the market for sale, but it typically doesn't include regular shops selling directly to people for their own personal use.
Why Should a Distributor Get Listed? (Eligibility and Importance)
Any company in Hong Kong with a properly staffed office that handles medical device distribution can apply to be added to "The List of Distributors." While it's a voluntary choice, and low-risk "Class I" medical devices aren't usually part of this listing (as they generally don't need device listing themselves), we strongly encourage distributors handling other types of devices (like Class II, III, IV general medical devices and Class B, C, D IVDMDs) to get listed.
What are the big benefits of being a listed medical device distributor?
- Credibility and Trust: Being on the list builds a lot of confidence with manufacturers, importers, hospitals, clinics, and even the end-users. It's a clear sign that you follow established standards.
- Market Preference: As more emphasis is placed on MDACS compliance, especially when public hospitals are buying equipment, our experience shows that listed distributors often gain a competitive edge.
- Better Tracking (Enhanced Traceability): The listing process and its requirements really help to track devices better throughout the distribution network. This is incredibly important for quickly responding to any safety concerns or recalls.
Key Things Listed Distributors Need to Do (Requirements)
To be listed under MDACS, a medical device distributor must set up, follow, and keep updated a set of written procedures for their operations. These procedures are absolutely fundamental, ensuring that devices stay safe and perform as they should throughout their journey through the distribution chain. Key areas include:
- Keeping Records of Transactions: Maintaining complete and up-to-date records of all distributed medical devices. From our perspective, these records need to have enough detail so you can trace devices back and forth, and quickly pull them from the market if needed. These records must be available for inspection by the MDD within a short, specific timeframe.
- Handling, Storage, and Delivery: Having clear, written steps for how medical devices are handled, stored, and delivered. Why is this so important? To protect devices from things like extreme temperatures or humidity that could affect their safety or performance. According to our experience, for IVDMDs, paying very close attention to temperature during storage and transport is especially critical.
- Managing Product Recalls and Safety Notices: Having clear procedures for effectively managing product recalls (when devices need to be taken back) and field safety notices (warnings about device issues). This means clear communication with manufacturers, LRPs, and customers, and a smooth process for getting back or fixing affected devices.
- Handling Reportable Incidents: Procedures for identifying and managing any serious or potentially serious incidents that need to be reported. Distributors are usually expected to work closely with the LRP or manufacturer to make sure these incidents are reported to the MDD as outlined in GN-03.
- Dealing with Complaints: Having a solid system for receiving, evaluating, investigating, and responding to any complaints about the medical devices they distribute.
- Maintenance and Service (If Applicable): If you also provide maintenance or service for the devices, you need written procedures for these activities too.
- Customer Feedback (If Applicable): Procedures for collecting feedback from customers about how the devices are performing.
The Application Process: What to Expect
From our experience, the application process to get listed as a medical device distributor in Hong Kong generally involves these steps:
- Submitting the Application Form: You'll submit the specific application form (often one that covers both importers and distributors, like MD-IP+D) through the online Medical Device Information System (MDIS). We generally recommend using MDIS as it streamlines the process.
- Providing Supporting Documents: You'll need to provide all the necessary supporting papers, including:
- A valid Business Registration Certificate for your Hong Kong operation.
- Details showing you have a properly staffed office in Hong Kong.
- Copies of all those written procedures we just talked about.
- Any other information or samples the MDD might ask for during their review.
- MDD Assessment: The MDD will review your application and all the documents. They might do assessments or ask for more information to make sure everything meets GN-09 and other MDACS requirements.
- Getting Listed: Once the assessment is successful, your company will be added to "The List of Distributors," which is publicly available on the MDD website. You'll also get an official listing certificate.
Ongoing Responsibilities: Staying on the List
From our perspective, getting listed is just the beginning. Once you're a listed medical device distributor in Hong Kong, you have an ongoing duty to continuously maintain and implement your documented procedures, keep your records accurate, and promptly tell the MDD about any relevant changes or incidents. Why is this adherence so vital? Because it ensures the continued safety and quality of medical devices as they travel through the supply chain to reach healthcare professionals and patients. This continuous effort is key to building lasting trust in the medical device market.
Ask Anything
We'll follow up with you personally.
Related Articles
Approximately 5 minutes
Medical Device Adverse Event Reporting in Hong Kong: A Guide for LRPs
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Medical Device Regulations and Registration in Hong Kong
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Navigating Medical Device Regulations in Hong Kong: The MDACS Framework
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.