Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 14, 2025
Approximately 5 minutes
Navigating Medical Device Regulations in Hong Kong: The MDACS Framework
Understanding the Medical Device Administrative Control System (MDACS) in Hong Kong: Our Insights
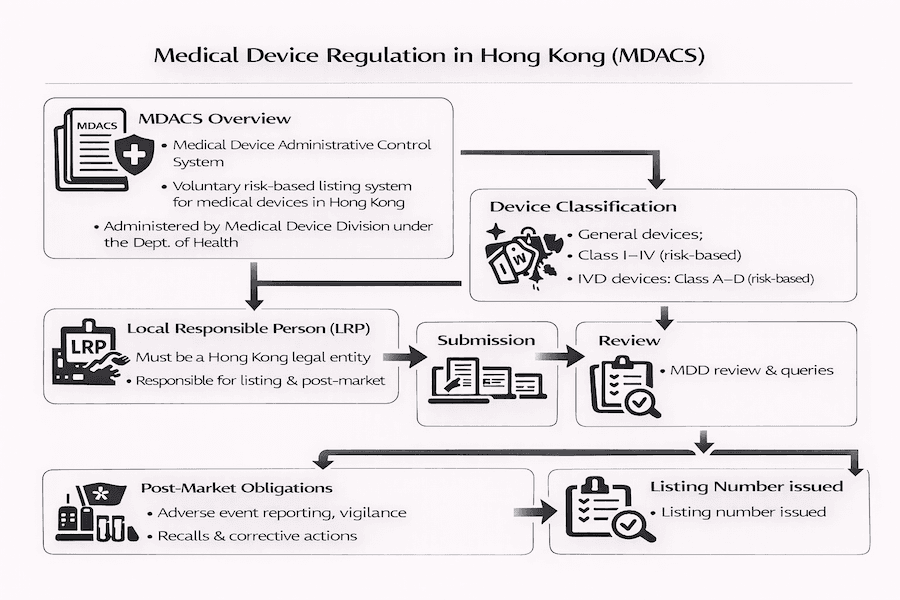
From what we've seen, Hong Kong has a unique way of managing medical devices, mainly through something called the Medical Device Administrative Control System (MDACS). The Department of Health (DH) launched this back in November 2004, and it's managed by their Medical Device Division (MDD). To our understanding, MDACS is built on a "risk-based" approach. What does that mean for you? It simply means the rules and checks become stricter as a device's potential risk goes up. Its main goal is to make sure all medical devices sold here are safe, good quality, and work properly.
According to our experience, this system follows international best practices, drawing ideas from global groups like the International Medical Device Regulators Forum (IMDRF). We see MDACS as a stepping stone, preparing the way for future, more formal laws about medical devices.
How are Medical Devices Classified in Hong Kong? Understanding Risk Categories
Under MDACS, medical devices are put into four different groups based on how much risk they might pose to patients and users. This classification is super important because it directly affects how much attention the regulators will give your device.
General Medical Devices:
- Class I (Low risk): These are the simplest devices with minimal risk. Think of everyday items like a tongue depressor or a basic bandage.
- Class II (Low-moderate risk): These have a bit more risk involved. Common examples include contact lenses or hearing aids.
- Class III (Moderate-high risk): We consider these higher-risk devices. This group includes things like ventilators used in hospitals or hip implants.
- Class IV (High risk): These are the most critical devices, where any malfunction could have very serious consequences. From our perspective, pacemakers and heart valves fall into this highest-risk category.
Similarly, In Vitro Diagnostic (IVD) medical devices (those used to test samples outside the body, like blood tests) are also categorized into Classes A, B, C, and D, from lowest to highest risk. The MDD provides helpful guides (called technical references like TR-003 for general devices and TR-006 for IVDs) to help manufacturers figure out the right class. This depends on things like what the device is used for, how long it's in contact with the body, if it involves surgery, and its potential for harm.
The Voluntary Listing System: Why It's Still Crucial
A key part of MDACS is its voluntary listing system for medium- to high-risk medical devices (that's Class II, III, IV general devices, and IVD Classes B, C, D). So, is listing mandatory? Not yet for all devices, but from what we've seen, it offers huge benefits. Public hospitals and other major buyers in Hong Kong increasingly prefer, and in many cases even require, devices that are listed under MDACS. According to our experience, this growing preference practically makes voluntary listing a must-do for genuinely getting your product into the Hong Kong market. Once a device is listed, its approval usually lasts for five years, so keeping an eye on renewal dates is important.
The Critical Role of the Local Responsible Person (LRP): Our Experience
For any manufacturer outside of Hong Kong who wants to sell medical devices here, appointing a Local Responsible Person (LRP) is an absolute requirement. Who is an LRP? Think of them as your vital local link between you, your importers, distributors, the people using your devices, and the Medical Device Division. From our experience, their responsibilities are quite extensive and crucial for smooth operations, including:
- Handling all the applications for getting your device listed.
- Making sure communication channels stay open and clear.
- Managing customer complaints and keeping track of where devices go (traceability).
- Reporting any changes made to listed devices.
- Most importantly, reporting adverse events (any unexpected problems or incidents) to the MDD. The MDACS has a built-in Adverse Event Reporting System, and from our perspective, it's vital for investigating incidents to prevent them from happening again and keeping the public safe.
The Listing Application Process: What to Expect from Our Experience
The general steps for getting your device voluntarily listed usually involve:
- Device Classification: First, you need to accurately figure out your device's risk class. We've found this initial step sets the stage for everything else.
- LRP Appointment: You then appoint a qualified LRP right here in Hong Kong.
- Dossier Compilation: This is where a lot of the detailed work happens. You (or your LRP) prepare a comprehensive set of application documents. According to our experience, making sure this "dossier" aligns with international submission guidelines (like those from GHTF/IMDRF) can really help.
- Submission and Review: Your LRP submits the application to the MDD for their review. From what we've seen, the MDD might ask for more information during this review, so prompt responses are key!
- Listing Approval: If everything looks good after the review, the MDD will give your device a unique listing number and add it to their public online database.
What if your device is already approved elsewhere? Manufacturers whose devices have already been approved in recognized "reference countries" (like the US, EU, Canada, Japan, Australia, Korea, or mainland China) might be able to go through a faster or simpler review process – which is a great advantage we often see utilized.
Market Access and the Greater Bay Area (GBA): Expanding Horizons
MDACS listing is becoming more and more important, not just for selling in Hong Kong itself, but also as a potential gateway to the exciting Guangdong-Hong Kong-Macao Greater Bay Area (GBA). Initiatives like the GBA Connected Scheme are, to our understanding, allowing medical devices listed in Hong Kong to be used in approved hospitals and clinics within the GBA. From our perspective, this opens up significant expansion opportunities for manufacturers, making Hong Kong an even more strategic entry point.
Future Outlook: Staying Ahead of the Curve
While MDACS currently operates as an administrative system, we know that Hong Kong has been seriously considering moving towards a mandatory legal framework for medical devices. This potential shift highlights the ongoing commitment to making regulatory oversight even stronger and ensuring the highest standards of medical device safety and quality in the region. Our advice to manufacturers and LRPs is to always stay updated with the latest guidelines and any new laws that come out, as this is vital for ensuring you remain compliant and can continue to access this dynamic market.
Ask Anything
We'll follow up with you personally.
Related Articles
Approximately 5 minutes
Medical Device Adverse Event Reporting in Hong Kong: A Guide for LRPs
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Medical Device Regulations and Registration in Hong Kong
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Navigating Medical Device Regulations in Hong Kong: The MDACS Framework
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.