ElendiLabs
虽然进口商有义务记录并向 LRP 报告质量问题,但根据 COP-01 守则,LRP 是法定的唯一沟通窗口。在发生严重事故(AE)或召回(FSCA)时,LRP 必须在 10 天内向 MDD 提交报告。 • 沟通路径: 列名进口商不能越过 LRP 直接处理行政程序。这种“独立 LRP + 商业进口商”的模式在 2026 年最为推荐,因为它能防止您的列名资格因更换经销商而失效,同时也确保了监管合规的专业性。
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
May 3, 2025
Approximately 5 minutes
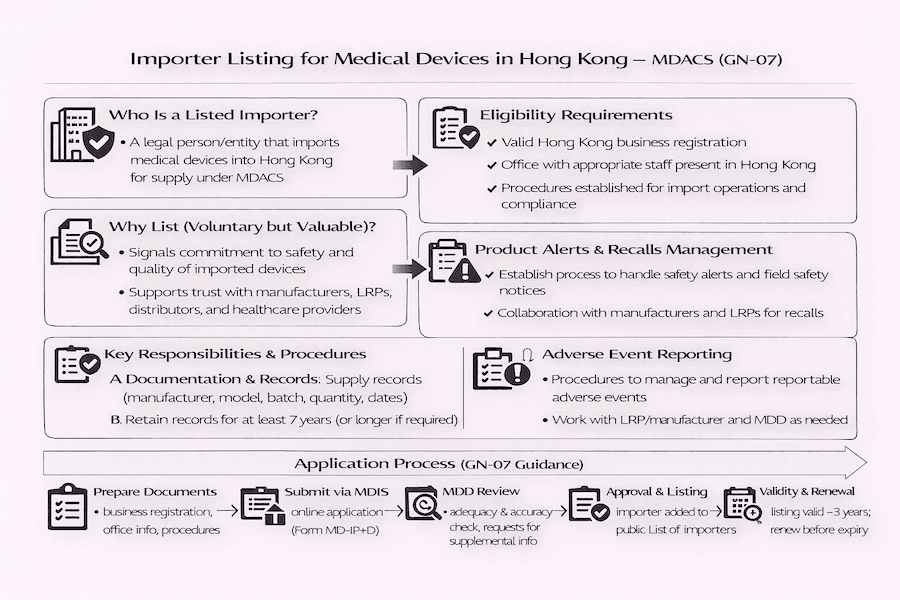
From our perspective, Hong Kong's Medical Device Administrative Control System (MDACS) isn't just about listing medical devices themselves; it also keeps a close eye on all the players in the supply chain. For companies that bring medical devices into Hong Kong, the "Guidance Notes for Listing of Importers of Medical Devices" (GN-07) clearly lays out the steps and requirements for getting voluntarily listed under this system. While it's not compulsory right now, choosing to be listed, in our experience, shows a strong commitment to quality and following the rules in the Hong Kong medical device market. It's a key step for any medical device importer Hong Kong.
When we talk about "medical devices" in Hong Kong, what exactly does the Department of Health's Medical Device Division (MDD) consider to be one? According to their guidelines, a medical device is essentially any instrument, apparatus, machine, appliance, implant, software, material, or similar item. It's important that the manufacturer intends it to be used, either alone or with other things, for specific medical purposes in humans.
These purposes include:
Crucially, a medical device does not achieve its main effect in or on the body through drugs, immune responses, or metabolism. However, it might be helped in its function by such means. So, it's pretty broad, covering everything from a simple bandage to complex diagnostic software!
So, what is an "Importer" in the world of MDACS, specifically for medical devices? Generally speaking, an "Importer" is a company or organization (a "legal entity") that either brings, or arranges to bring, any medical devices covered by MDACS into Hong Kong for sale or supply here. From our understanding, this definition usually doesn't include individuals who simply import devices for their own personal use. This distinction is vital for medical device regulation Hong Kong.
Any legal company with a properly staffed office in Hong Kong that handles the import of medical device(s) can apply to be included in "The List of Importers." According to our experience, the voluntary listing of importers offers several clear advantages:
To get listed under MDACS, an importer needs to set up, follow, and keep up-to-date a set of written procedures. What's the big picture here? These procedures are absolutely vital for properly controlling and managing imported medical devices, ensuring their safety and quality from arrival to distribution. They should cover important areas like:
Ready to apply? The process for getting listed as a medical device importer in Hong Kong typically involves these steps, based on our guidance to clients:
Getting listed as a medical device importer isn't a one-time event; it's an ongoing commitment. According to our experience, listed importers are expected to continuously keep their written procedures in place and follow them, keep their records updated, and quickly tell the MDD about any important changes or incidents. This continuous dedication is really important for keeping the medical device supply chain reliable and ensuring public health protection here in Hong Kong. It's about maintaining trust and safety in the Hong Kong healthcare market for the long run.
We'll follow up with you personally.
ElendiLabs
虽然进口商有义务记录并向 LRP 报告质量问题,但根据 COP-01 守则,LRP 是法定的唯一沟通窗口。在发生严重事故(AE)或召回(FSCA)时,LRP 必须在 10 天内向 MDD 提交报告。 • 沟通路径: 列名进口商不能越过 LRP 直接处理行政程序。这种“独立 LRP + 商业进口商”的模式在 2026 年最为推荐,因为它能防止您的列名资格因更换经销商而失效,同时也确保了监管合规的专业性。
Anonymous
我们在香港有三家不同的经销商,分别负责公立医院、私立诊所和零售药店。根据 GN-07 指南,我们可以将这三家全部登记为‘列名进口商’吗?如果其中一家进口商因为违反《医疗器械实务守则》(Code of Practice) 被除名,是否会连带影响我们的产品表列号 (HKMD No.) 或其他两家进口商的进口资格?
ElendiLabs
您可以授权无限数量的列名进口商。通过 LRP 在 MDIS (医疗器械信息系统) 门户操作,只需提交每家进口商的商业登记证 (BR) 及对应的授权书。 连带影响: 2026 年的监管趋于精准化。单个进口商的违规(如仓储温控不达标)通常只会导致该特定进口商从您的产品表列名单中移除,不会自动撤销产品的 HKMD 编号。但是,如果违规涉及系统性质量问题(如篡改标签),MDD 可能会要求 LRP 解释,严重时会暂停整个产品的进口。
Anonymous
我们目前的进口商仅具备基础的商业登记。面对即将立法的 CMPR 框架,进口商是否必须获得 ISO 13485 认证?
ElendiLabs
目前 MDACS 仅要求进口商遵循“Code of Practice”。但 2026 年 CMPR 预览版建议,处理 Class III/IV 高风险设备的进口商需建立符合 ISO 13485 (或同等标准) 的质量管理体系。
Approximately 5 minutes
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.
Approximately 5 minutes
This article details the application process for listing Class B, C, and D In Vitro Diagnostic Medical Devices (IVDMDs) under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-06. It covers classification, eligibility, submission requirements, and the online application via MDIS, all based on our insights and experience for efficient IVDMD listing in Hong Kong.
Anonymous
我们计划聘请 ElendiLabs 作为独立 LRP 以掌握产品所有权,但我们的商业合作伙伴希望作为列名进口商。在2026年的合规要求下,如果发生产品召回,由谁负责向卫生署医疗器械分部 (MDD) 提交初步报告?进口商是否可以直接与 MDD 沟通,还是必须通过我们的 LRP?