Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 16, 2025
Approximately 5 minutes
Managing Changes for Listed Medical Devices in Hong Kong: A Guide to GN-10
Navigating Changes for Listed Medical Devices in Hong Kong: Our Experience with GN-10
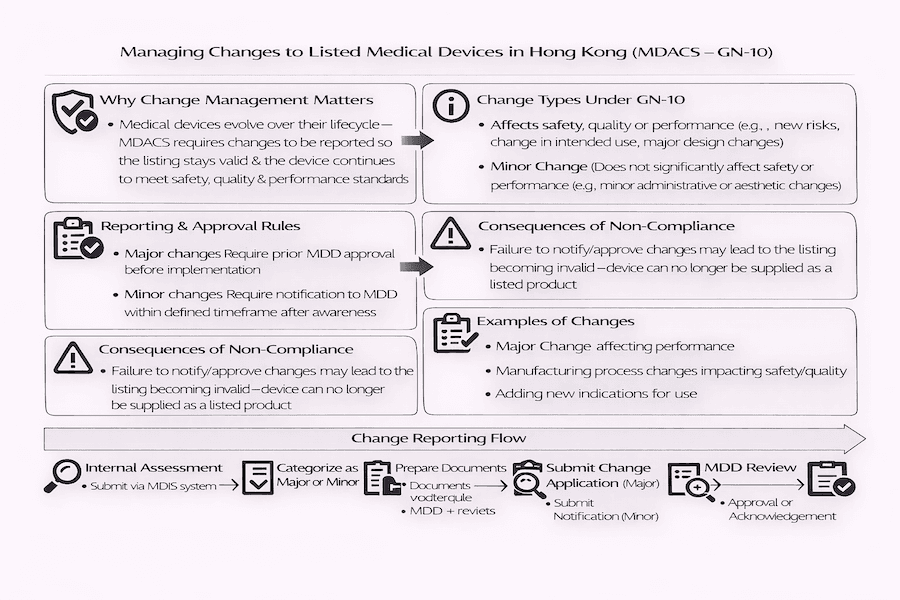
From our perspective, just getting a medical device listed in Hong Kong isn't the end of the journey. Medical devices, much like any other sophisticated product, are bound to evolve and undergo various updates throughout their lifespan. Under Hong Kong's Medical Device Administrative Control System (MDACS), it's absolutely crucial that any changes made to devices already on the official list are handled correctly and reported. This, as we understand it, ensures they continue to meet all the safety and performance rules.
The "Guidance Notes on Changes for Listed Medical Devices" (GN-10) acts as a comprehensive playbook for Local Responsible Persons (LRPs). It clearly shows them how to identify, manage, and report these changes to the Medical Device Division (MDD) of the Department of Health.
Why is Managing Changes So Important?
The main reason for GN-10, as we've learned, is to protect public health. It ensures that the information about listed medical devices in the MDACS database always stays accurate and up-to-date. Why does this matter? Because any change to a device must not mess with its basic safety and how well it works. By reporting changes in a timely manner, the MDD can keep accurate records and check for any potential new risks to patients, users, or anyone else involved. According to our experience, failing to tell the MDD about changes within the set timeframes, or not getting their green light for big changes beforehand, can actually make the device's listing invalid! This is a serious consequence we always advise clients to avoid.
Categorizing Changes: Is it a Big Deal or a Small Tweak?
GN-10 divides changes into two main types, based on how much they might affect a medical device's safety, quality, or performance (SQP). How do we decide if a change is 'major' or 'minor'?
-
Major Change (需要事先批准的大變動):
- This is a change that, from our perspective, could directly impact the device's safety, quality, or how well it performs.
- Typically, a major change might:
- Lead to risks for the patient that we haven't seen before.
- Increase the chances of existing problems happening.
- Change how existing or new risks are presented to the user (for example, if the labels need big changes, or the device is now used for something new).
- What are some examples of major changes we've encountered? These often include changes to what the device is used for, its design, how it's made, how it's sterilized, crucial parts, or big changes to its software that could affect safety or performance.
- Major changes absolutely need prior approval from the MDD before you can put them into practice. This is a non-negotiable step.
-
Minor Change (只需通知的小調整):
- This is a change that, in our assessment, doesn't fit the definition of a Major Change.
- Minor changes usually don't have a big impact on the device's safety, quality, or performance, and they don't introduce new risks.
- What are some common minor changes we see? These could be simple administrative updates, small cosmetic tweaks, or updates to parts that aren't critical to the device's main function.
- For minor changes, you generally need to notify the MDD within a specific timeframe (for example, 24 weeks from when the LRP becomes aware of the change), but you usually don't need their approval beforehand.
GN-10 often includes helpful flowcharts. According to our experience, these flowcharts are invaluable for LRPs when trying to figure out if a change is major or minor. A key point to remember: if you're making several changes at once, and even one of them is considered a major change, then all those simultaneous changes are treated as a collective Major Change.
The Crucial Role of the Local Responsible Person (LRP)
The LRP, in our view, plays a truly central role in managing any changes to listed medical devices. Their responsibilities include:
- Assessment: Carefully checking how the change might affect the patient, the doctor or nurse using it, and the device's overall specifications.
- Determination: Using GN-10 to decide if the change is a Major Change or a Minor Change.
- Notification/Application: Submitting the right form (an application for major changes, or just a notification for minor changes) to the MDD within the required time. This typically involves using a specific Change Application Form.
- Documentation: Keeping very thorough records of all changes, why they were categorized that way, and proof that the device still meets its basic safety and performance requirements.
- Compliance: Making sure the updated medical device continues to comply with all the "Essential Principles of Safety and Performance."
The Change Application/Notification Process: What to Expect
From our experience, the process generally unfolds like this:
- Internal Assessment: The LRP, working closely with the manufacturer, does a detailed check of the change and decides if it's a major or minor one.
- Preparing Documents: All necessary supporting documents for the change are put together, including updated technical files, risk analyses, and validation reports.
- Submitting to MDD:
- For Major Changes: You submit a Change Application Form to the MDD for their pre-approval. The MDD will review it, and the change can only be put into effect after they've given their approval.
- For Minor Changes: You simply notify the MDD by submitting a Change Application Form within the set notification period.
- MDD Review and Approval/Acknowledgement: The MDD will look over the information you've submitted. For major changes, they'll either approve it or ask for more details. For minor changes, they'll simply confirm they've received your notification.
Keeping Your Listing Valid: A Critical Reminder
It's absolutely essential for LRPs to stick strictly to the rules in GN-10. Why is this so important? Because if a Major Change is made without getting the MDD's approval first, or if any changes aren't reported within the given timeframe, the medical device's listing could immediately become invalid. To our understanding, in such cases, the device would no longer be considered officially listed under MDACS. This means the LRP would have to stop selling the medical device in a way that suggests it's still listed.
By carefully following GN-10, LRPs and manufacturers ensure that medical devices in Hong Kong's active market remain safe and meet all the necessary rules, which ultimately protects patients.
Ask Anything
We'll follow up with you personally.
Related Articles
Approximately 5 minutes
Medical Device Adverse Event Reporting in Hong Kong: A Guide for LRPs
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Medical Device Regulations and Registration in Hong Kong
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Navigating Medical Device Regulations in Hong Kong: The MDACS Framework
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.
Approximately 5 minutes
Listing Procedures for Local Medical Device Manufacturers in Hong Kong: A Guide to GN-08
This article details the application process for local medical device manufacturers seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-08. It covers eligibility, quality management system requirements, and the submission process, all based on our insights and experience for effective Hong Kong medical device manufacturing.
Approximately 5 minutes
Listing Procedures for Medical Device Importers in Hong Kong: A Guide to GN-07
For entities importing medical devices into Hong Kong, the Medical Device Administrative Control System (MDACS) provides a voluntary listing scheme for importers, guided by GN-07. This article details the eligibility, application steps, and key requirements for listing as a medical device importer, based on our insights and experience for efficient Hong Kong medical device import.
Approximately 5 minutes
Listing Procedures for Medical Device Distributors in Hong Kong: A Guide to GN-09
This article outlines the application process for medical device distributors seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as detailed in GN-09. It covers eligibility, key requirements for documented procedures, and the submission process to enhance traceability and public safety, based on our insights.
Approximately 5 minutes
Listing Procedures for Class B, C, and D In Vitro Diagnostic Medical Devices in Hong Kong: A Guide to GN-06
This article details the application process for listing Class B, C, and D In Vitro Diagnostic Medical Devices (IVDMDs) under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-06. It covers classification, eligibility, submission requirements, and the online application via MDIS, all based on our insights and experience for efficient IVDMD listing in Hong Kong.