Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
April 17, 2025
Approximately 5 minutes
Essential Principles of Safety and Performance for Medical Devices in Hong Kong: Understanding TR-001 and TR-004
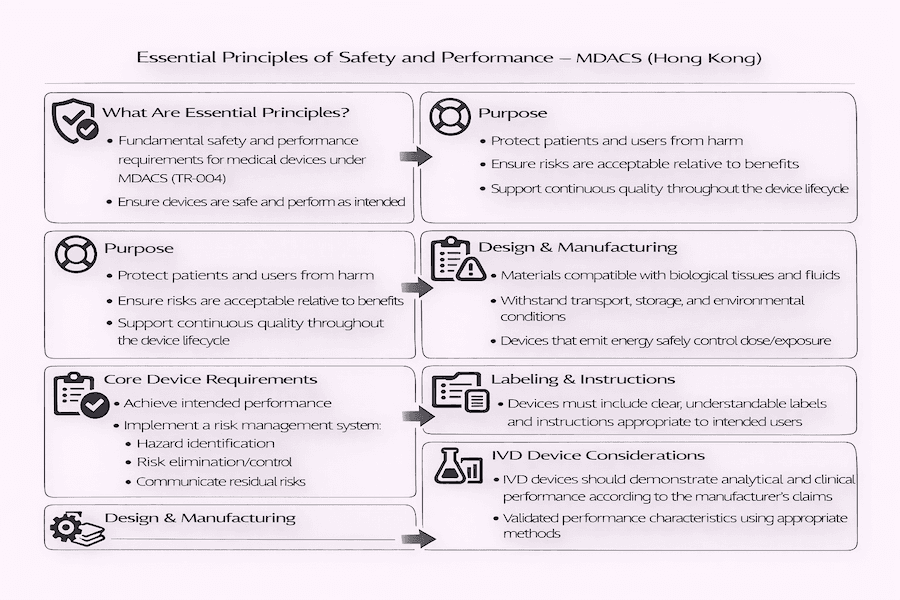
The Cornerstone of Medical Device Safety: Hong Kong's Essential Principles – Our Insights
From what we've observed, ensuring the safety and performance of medical devices in Hong Kong's market is absolutely paramount. This commitment is built upon a set of fundamental requirements known as the Essential Principles of Safety and Performance of Medical Devices. These important principles are mainly explained in Technical Reference TR-004. At the same time, Technical Reference TR-001 describes how manufacturers can actually prove they meet these principles, outlining the evidence and procedures they need to follow.
To our understanding, these documents aren't just made up out of thin air. They're adapted directly from international best practices, especially the recommendations from groups like the International Medical Device Regulators Forum (IMDRF). This ensures that Hong Kong's standards for medical device safety Hong Kong are right in line with what's expected globally.
Overarching Aim: Safety and Intended Performance – What's the Big Goal?
What's the core idea behind all these Essential Principles? Simply put, their main goal is to make sure that medical devices are designed and made in such a way that, when you use them as intended and under normal conditions, they won't put patients, users, or anyone else at risk. Any risks that do exist must be acceptable when you compare them to the benefits the patient gets, and according to our experience, these risks should be reduced as much as reasonably possible.
Manufacturers, we've found, are expected to use a "risk management" approach. This means they should prioritize eliminating or reducing potential dangers right from the design stage and throughout the manufacturing process, making devices inherently safe. This is crucial for regulatory compliance Hong Kong.
Key Areas Covered by the Essential Principles: A Closer Look
The Essential Principles cover a wide range of requirements that can be generally grouped into several important areas:
-
General Requirements: The Basics for Safe Devices
- Risk Management: Manufacturers must have a clear plan for managing risks. What does this involve? Identifying known or foreseeable dangers, figuring out how big those risks are, and then either getting rid of them or controlling them. Users must also be told about any remaining risks. This is central to Hong Kong medical device regulation.
- Benefit-Risk Ratio: The good things the device does must always outweigh any potential harms.
- Performance and Intended Purpose: The device needs to work exactly as the manufacturer says it will, and it must be suitable for its intended use under normal conditions.
- Lifetime Performance: The device's features and how well it works shouldn't get worse during its expected lifespan, as stated by the manufacturer.
- Transport and Storage: Devices must be designed, made, and packaged in a way that ensures their characteristics and performance aren't negatively affected during shipping and storage.
- Stability: The device should remain stable and effective during its shelf-life and after it's opened for use.
-
Design and Manufacturing Requirements: Building Safety In
- Chemical, Physical, and Biological Properties: Devices must be designed and made from materials that keep risks from contaminants and residues to a minimum. Being compatible with the body's tissues, fluids, and other substances is also vital.
- Infection and Microbial Contamination: Design and manufacturing processes should eliminate or greatly reduce the risk of infection for patients and users, especially for devices that are supplied sterile.
- Manufacturing and Environmental Properties: Devices must be designed for safe use with any materials, substances, and gases they might come into contact with. Risks from substances leaching out or leaking from the device, or unwanted entry/exit of substances, should be minimized.
- Devices with a Measuring Function: Devices meant to measure things (like blood pressure monitors or thermometers) must be designed and made to be accurate, precise, and stable enough for what they're supposed to do.
- Protection against Radiation: Devices that give off radiation must be designed and manufactured to keep exposure for patients, users, and others as low as possible, consistent with how the device is meant to be used.
- Protection against Electric Shock, Mechanical, and Thermal Risks: Devices must protect against dangers from electricity, moving parts, and heat.
- Protection against Risks to Patients or Users: This covers things like how the device gets power, if it has alarm systems, and how it prevents accidental misuse.
Do all medical devices need to meet all of these design and manufacturing principles? Not necessarily. Manufacturers only need to apply the Essential Principles that are relevant to their specific device. For example, a non-electrical bandage wouldn't need to meet requirements for protection against electric shock. The key is applying a risk-based approach to determine relevancy.
-
Information Supplied by the Manufacturer: Clear Communication is Key
- Labeling and Instructions for Use (IFU): Crucial information must be provided directly on the device or its packaging, and in easy-to-understand instructions for use. What sort of information? This includes the device name, manufacturer details, its intended use, any important warnings, and clear instructions for safe use.
- Unique Device Identification (UDI): Where applicable (and we're seeing this become more common globally), devices should have a UDI to make them easier to trace. This helps with medical device traceability Hong Kong.
Proving Conformity: The Role of TR-001 in Practice
While TR-004 gives you the list of Essential Principles, TR-001 is where we really get into the nitty-gritty of proving you meet them. It describes the evidence and procedures manufacturers use to show that their medical devices are safe, perform as intended, and fully conform to these Essential Principles. What kind of evidence are we talking about? This includes details about your quality management system (how you manage quality), your post-market surveillance system (how you keep an eye on the device after it's sold), your summary technical documentation (STED – a concise summary of all your technical info), and your declaration of conformity (a formal statement that you meet the rules).
According to our experience, the more risk a device poses, the tougher this "conformity assessment" becomes. This directly influences medical device registration Hong Kong.
Our Final Take: Beyond Just Regulations
Adherence to these Essential Principles isn't just about ticking boxes for regulators. From our perspective, it's a fundamental commitment from manufacturers to ensuring patient safety and confirming their product actually works as it should within Hong Kong's dynamic medical device market. And the LRPs? They play an absolutely vital role in making sure these principles are upheld throughout the device's entire life cycle. Are you confident your medical device fully meets these essential principles for the Hong Kong market?
Ask Anything
We'll follow up with you personally.
Related Articles
Approximately 5 minutes
Medical Device Adverse Event Reporting in Hong Kong: A Guide for LRPs
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Medical Device Regulations and Registration in Hong Kong
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Navigating Medical Device Regulations in Hong Kong: The MDACS Framework
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.
Approximately 5 minutes
Listing Procedures for Local Medical Device Manufacturers in Hong Kong: A Guide to GN-08
This article details the application process for local medical device manufacturers seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-08. It covers eligibility, quality management system requirements, and the submission process, all based on our insights and experience for effective Hong Kong medical device manufacturing.
Approximately 5 minutes
Listing Procedures for Medical Device Importers in Hong Kong: A Guide to GN-07
For entities importing medical devices into Hong Kong, the Medical Device Administrative Control System (MDACS) provides a voluntary listing scheme for importers, guided by GN-07. This article details the eligibility, application steps, and key requirements for listing as a medical device importer, based on our insights and experience for efficient Hong Kong medical device import.
Approximately 5 minutes
Listing Procedures for Medical Device Distributors in Hong Kong: A Guide to GN-09
This article outlines the application process for medical device distributors seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as detailed in GN-09. It covers eligibility, key requirements for documented procedures, and the submission process to enhance traceability and public safety, based on our insights.
Approximately 5 minutes
Listing Procedures for Class B, C, and D In Vitro Diagnostic Medical Devices in Hong Kong: A Guide to GN-06
This article details the application process for listing Class B, C, and D In Vitro Diagnostic Medical Devices (IVDMDs) under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-06. It covers classification, eligibility, submission requirements, and the online application via MDIS, all based on our insights and experience for efficient IVDMD listing in Hong Kong.