Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 23, 2025
Approximately 5 minutes
Essential Principles of Safety and Performance for Medical Devices in Hong Kong: A Deep Dive into TR-004
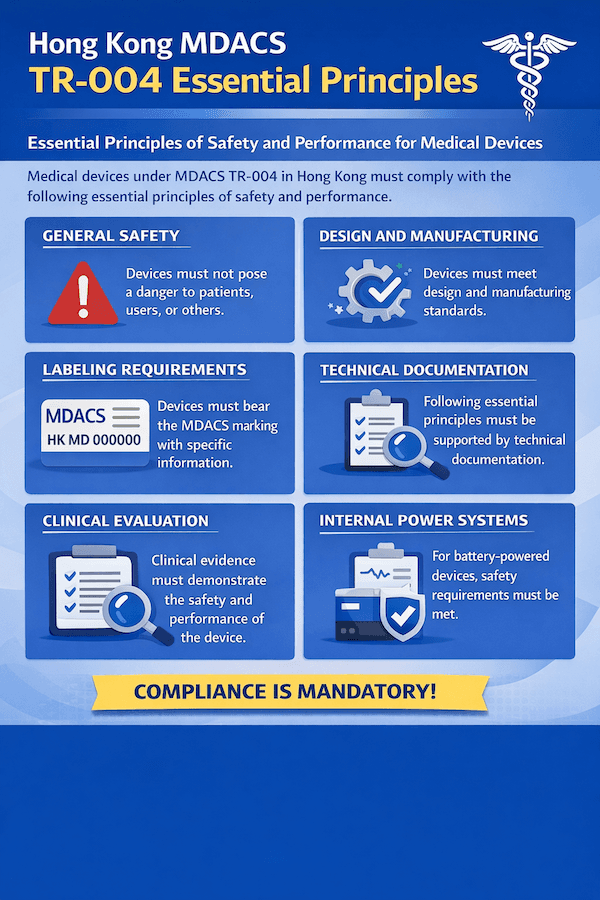
Unpacking the Essential Principles: TR-004's Mandate for Medical Devices in Hong Kong – Our Insights
From what we've observed, the safety and effectiveness of medical devices circulating in Hong Kong are rigorously upheld through something called the Essential Principles of Safety and Performance of Medical Devices. These are laid out in great detail in Technical Reference TR-004, which is part of the Medical Device Administrative Control System (MDACS). To our understanding, this document is a truly critical guide for manufacturers, Local Responsible Persons (LRPs), and everyone else involved. It spells out the basic things medical devices must do throughout their entire life – from when they're first designed and made, all the way to how they're monitored after they're sold.
According to our experience, TR-004 isn't just a local rulebook; it's designed to align with international best practices. It takes direct guidance from groups like the IMDRF (International Medical Device Regulators Forum). What's its main goal? Simply put, it aims to make sure that when medical devices are used as intended, they won't harm patients, or the people using them. Any risks involved must be acceptable when you weigh them against the benefits, and crucially, they should be reduced as much as reasonably possible. This is paramount for medical device safety Hong Kong.
Core Philosophy: Risk Management and Safety by Design – What's the Big Idea Here?
A central theme that runs right through TR-004 is the idea of proactively managing risks. What does that really mean for manufacturers? Fundamentally, they are expected to:
- Identify known or foreseeable hazards: Think about what could potentially go wrong and estimate the risks involved.
- Eliminate risks: Wherever reasonably possible, risks should be designed out of the product from the very beginning. This is about building safety in, right from the manufacturing process.
- Reduce remaining risks: If you can't eliminate a risk completely, then you need to take good measures to reduce it to an acceptable level.
- Inform users: Make sure everyone who uses the device knows about any risks that couldn't be completely removed.
From our perspective, this step-by-step approach prioritizes safety right from the drawing board. It's about ensuring "safety by design" as a foundational principle, which is key for regulatory compliance Hong Kong.
Key Aspects of the Essential Principles (as per TR-004): Let's Break Them Down
TR-004 groups these Essential Principles into several big categories, each with its own specific rules. What are the main areas you should focus on?
-
General Requirements: The Non-Negotiables for Performance and Safety
- Performance and Safety: Your device must perform as you say it will, and it must be designed and made to be safe for its intended use. Any risks have to be acceptable when compared to the benefits it offers.
- Risk Control: Manufacturers need to apply strong safety principles, always keeping in mind the latest and best industry practices, to get rid of or reduce risks. This is critical for risk management medical devices Hong Kong.
- Performance During Lifetime: The device should keep its performance and characteristics throughout its expected useful life. It shouldn't degrade unexpectedly.
- Transport and Storage: How your device is designed, made, and packaged needs to ensure that shipping and storage won't mess with its features or performance.
- Stability: It needs to be stable during its shelf-life, and also when it's being used after opening (especially important for IVDs), and during transport.
- Undesirable Side-effects: All known and potential negative side effects should be kept to a minimum and be acceptable compared to the benefits.
-
Design and Manufacturing Requirements: Building Safety from the Ground Up
- Chemical, Physical, and Biological Properties: This gets into the nitty-gritty of the materials used. They need to be compatible with the body (biocompatibility), minimize harmful contaminants, and safely interact with any substances or gases.
- Infection and Microbial Contamination: Design and manufacturing processes must aim to eliminate or reduce the risk of infection for patients and users. If your device is supplied sterile, proving that sterility is maintained is crucial.
- Devices with a Measuring Function: For devices that measure things (like blood pressure or temperature), they have specific rules for accuracy, precision, and stability. Is your device giving consistently reliable readings? It needs to.
- Protection against Radiation: If your device gives off any radiation, it must be designed and made to keep exposure to patients, users, and others as low as possible, consistent with its purpose.
- Protection against Electric Shock, Mechanical, and Thermal Risks: Your device needs to be safe from electrical hazards, strong enough mechanically, and not overheat.
- Protection against Risks to Patients or Users: This covers things like how the device gets power, if it has alarm systems, how it prevents accidental misuse, and even how comfortable and easy it is to use (ergonomics).
Do all medical devices need to meet every single one of these design and manufacturing principles? From our experience, not necessarily! Manufacturers only need to apply the Essential Principles that are genuinely relevant to their specific device. For example, a simple, non-electrical bandage wouldn't need to meet requirements for protection against electric shock. The key is using a smart, risk-based approach to figure out what applies. This is important for medical device registration Hong Kong.
-
Information Supplied by the Manufacturer: Clear Communication is Non-Negotiable
- Labelling and Instructions for Use (IFU): Your device must come with all the necessary information so people can use it safely and properly. This includes identifying the device and, where relevant, tracing it back. You'll need detailed instructions, warnings, and precautions.
- Specific Labelling Elements: This includes vital details like the manufacturer's name and address, the device name, its batch or lot number, an expiry date (if it has one), and clear instructions if it's a single-use device. Remember, TR-005 also adds specific Hong Kong labelling requirements that are super important to follow!
Demonstrating Conformity: How Do You Prove It Works and Is Safe?
Manufacturers are ultimately responsible for proving that their medical devices actually comply with all the applicable Essential Principles. How do you do this? It involves creating objective evidence throughout the device's entire lifecycle. According to our experience, the higher the risk class of the device, the more thorough and rigorous this "conformity assessment" process becomes. This evidence forms the backbone of your technical documentation, including the Summary Technical Documentation (STED) (which is outlined in TR-002) that you submit for MDACS listing.
Our Final Take: Beyond Just Regulations, It's About Trust
Adherence to these Essential Principles isn't just about jumping through regulatory hoops. From our perspective, it's a fundamental commitment from manufacturers to ensuring patient safety and confirming their product truly delivers on its promise within Hong Kong's dynamic medical device market. And the LRPs? They play an absolutely vital role in making sure these principles are upheld consistently throughout the device's entire life cycle. Are you confident your medical device fully meets these essential principles for the Hong Kong market, building trust with users and regulators alike?
Ask Anything
We'll follow up with you personally.
Related Articles
Approximately 5 minutes
Medical Device Adverse Event Reporting in Hong Kong: A Guide for LRPs
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Medical Device Regulations and Registration in Hong Kong
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Navigating Medical Device Regulations in Hong Kong: The MDACS Framework
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.
Approximately 5 minutes
Listing Procedures for Local Medical Device Manufacturers in Hong Kong: A Guide to GN-08
This article details the application process for local medical device manufacturers seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-08. It covers eligibility, quality management system requirements, and the submission process, all based on our insights and experience for effective Hong Kong medical device manufacturing.
Approximately 5 minutes
Listing Procedures for Medical Device Importers in Hong Kong: A Guide to GN-07
For entities importing medical devices into Hong Kong, the Medical Device Administrative Control System (MDACS) provides a voluntary listing scheme for importers, guided by GN-07. This article details the eligibility, application steps, and key requirements for listing as a medical device importer, based on our insights and experience for efficient Hong Kong medical device import.
Approximately 5 minutes
Listing Procedures for Medical Device Distributors in Hong Kong: A Guide to GN-09
This article outlines the application process for medical device distributors seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as detailed in GN-09. It covers eligibility, key requirements for documented procedures, and the submission process to enhance traceability and public safety, based on our insights.
Approximately 5 minutes
Listing Procedures for Class B, C, and D In Vitro Diagnostic Medical Devices in Hong Kong: A Guide to GN-06
This article details the application process for listing Class B, C, and D In Vitro Diagnostic Medical Devices (IVDMDs) under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-06. It covers classification, eligibility, submission requirements, and the online application via MDIS, all based on our insights and experience for efficient IVDMD listing in Hong Kong.