Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 23, 2025
Approximately 5 minutes
Classification of General Medical Devices in Hong Kong: A Guide to TR-003
Categorizing Safety: General Medical Device Classification in Hong Kong – Our Insights
From what we've observed, Hong Kong's Medical Device Administrative Control System (MDACS) uses a clever system to classify medical devices based on their risk. This is all about making sure that the right amount of regulatory attention is given to each device. For general medical devices, all the specific rules and principles for classification are clearly laid out in Technical Reference TR-003, which is titled "Classification of General Medical Devices." To our understanding, this document is truly vital for manufacturers and Local Responsible Persons (LRPs) because it directly affects what kind of "conformity assessment" (proving your device meets standards) you'll need and how your entire listing process under MDACS will go. This is a foundational step for medical device classification Hong Kong.
According to our experience, the classification principles in TR-003 aren't just local ideas; they match international guidelines perfectly, especially those from the International Medical Device Regulators Forum (IMDRF). This really helps keep things consistent globally when it comes to medical device regulation Hong Kong.
The Four Risk Classes: How Risky is Your Device?
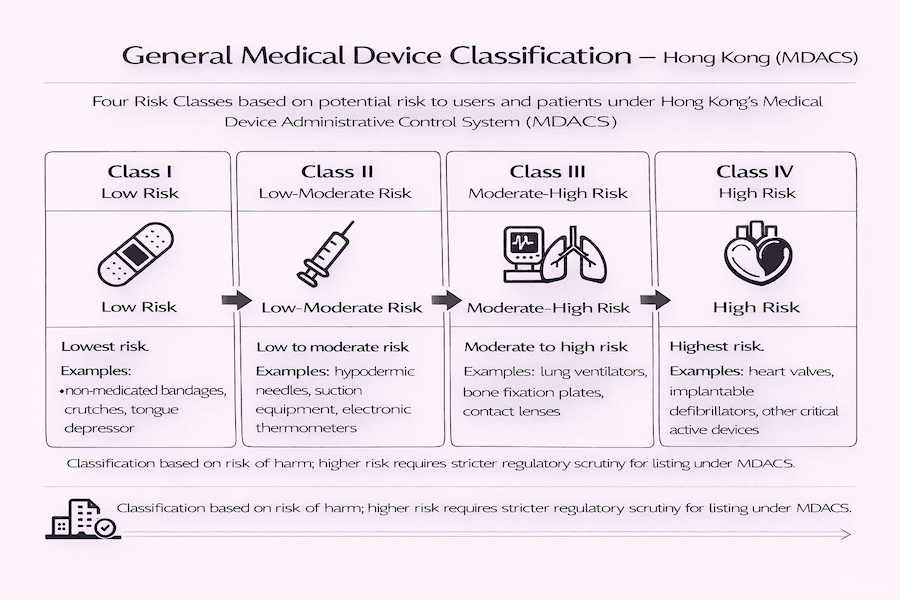
Have you ever wondered how medical devices are categorized? General medical devices under MDACS are put into four different classes. These classes range from the lowest risk to the highest, and what does that mean for you? The higher the risk, the more scrutiny your device will face.
- Class I (低風險): These are the lowest risk devices. Think of simple, everyday items like non-medicated bandages, crutches, or a tongue depressor. To our understanding, these generally don't need listing under MDACS.
- Class II (中低風險): These carry a low-to-moderate risk. Common examples we see are hypodermic needles, suction equipment, or electronic thermometers.
- Class III (中高風險): These devices are considered moderate-to-high risk. This group includes things like lung ventilators, bone fixation plates, or contact lenses.
- Class IV (高風險): These are the highest risk devices. We're talking about critical items like heart valves, implantable defibrillators, or other active devices that go inside the body.
According to our experience, the higher your device's risk class, the stricter the regulatory checks will be, and the more extensive the evidence you'll need to provide for listing.
Principles of Classification: What Determines Your Device's Category?
TR-003 outlines a series of rules for classifying devices. So, what actually determines where your specific device fits in? Its classification depends on one or several factors related to its design and what it's intended to be used for. These factors can, either alone or in combination, affect your device's classification:
- Duration of Contact with the Body: How long does your device touch the body? Is it just for a moment (transient), a short time, or a long time?
- Degree of Invasiveness: Does your device break the skin or go inside the body? Is it non-invasive, invasive (like entering a body opening or through surgery), or actually implanted?
- Delivery of Medicinal Products or Energy: Does your device deliver medicine or energy? And what kind of delivery is it?
- Biological Effect or Systemic/Local Effects: Is your device meant to have a biological effect (like interacting with cells) or be absorbed by the body?
- Use in Combination with other Medical Devices: If your device is designed to be used with another medical device, its classification might be influenced by how they work together.
- Standalone Software: Is your software a medical device on its own? If it qualifies, its classification depends on its own intended use and the risks it brings. Standalone software is usually considered an active device.
Application of Classification Rules: Our Practical Approach
TR-003 gives you a step-by-step way to apply these classification rules. What's our advice for doing this effectively?
- Step-by-step Evaluation: We always guide manufacturers to carefully evaluate their device against each rule in a systematic way.
- Highest Class Principle: What if multiple rules apply? This is a common question! If your device could fit into more than one category, the rule that leads to the highest classification (meaning the greatest risk) is the one you must use. From our perspective, this conservative approach is designed to ensure that your device is regulated under the strictest possible standards, prioritizing patient safety.
- Examples and Explanations: The document itself provides helpful examples of classified general medical devices to show you how the rules work in practice. However, it's important to remember, as we always emphasize, that the final classification of your specific device really depends on its actual design, what you intend it to be used for, and other unique factors. Don't just rely on examples; dig into your specific product details.
Manufacturer's Responsibility and LRP's Role: Partnering for Success
The manufacturer is ultimately responsible for correctly classifying their medical device according to the rules in TR-003. We strongly encourage you to document why you assigned your product to a particular risk class. This rationale can be very helpful during the review process.
The Local Responsible Person (LRP) in Hong Kong, from our experience, plays a truly crucial role in helping foreign manufacturers with this classification. They make sure that the listing application submitted accurately reflects the device's correct risk class. Why is correct classification so foundational? Because it's the very basis for meeting all the subsequent conformity assessment requirements and for successfully navigating the entire MDACS listing process. To our understanding, misclassification can lead to significant delays in approval or even non-compliance, which no one wants!
By diligently applying the principles and rules in TR-003, manufacturers contribute to ensuring that medical devices placed on the Hong Kong market are appropriately regulated, promoting patient safety and public health.
Ask Anything
We'll follow up with you personally.
Related Articles
Approximately 5 minutes
Medical Device Adverse Event Reporting in Hong Kong: A Guide for LRPs
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Medical Device Regulations and Registration in Hong Kong
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Navigating Medical Device Regulations in Hong Kong: The MDACS Framework
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.
Approximately 5 minutes
Listing Procedures for Local Medical Device Manufacturers in Hong Kong: A Guide to GN-08
This article details the application process for local medical device manufacturers seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-08. It covers eligibility, quality management system requirements, and the submission process, all based on our insights and experience for effective Hong Kong medical device manufacturing.
Approximately 5 minutes
Listing Procedures for Medical Device Importers in Hong Kong: A Guide to GN-07
For entities importing medical devices into Hong Kong, the Medical Device Administrative Control System (MDACS) provides a voluntary listing scheme for importers, guided by GN-07. This article details the eligibility, application steps, and key requirements for listing as a medical device importer, based on our insights and experience for efficient Hong Kong medical device import.
Approximately 5 minutes
Listing Procedures for Medical Device Distributors in Hong Kong: A Guide to GN-09
This article outlines the application process for medical device distributors seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as detailed in GN-09. It covers eligibility, key requirements for documented procedures, and the submission process to enhance traceability and public safety, based on our insights.
Approximately 5 minutes
Listing Procedures for Class B, C, and D In Vitro Diagnostic Medical Devices in Hong Kong: A Guide to GN-06
This article details the application process for listing Class B, C, and D In Vitro Diagnostic Medical Devices (IVDMDs) under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-06. It covers classification, eligibility, submission requirements, and the online application via MDIS, all based on our insights and experience for efficient IVDMD listing in Hong Kong.