Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 23, 2025
Approximately 5 minutes
Medical Device Registration in Hong Kong: Essential Requirements and Procedures
Medical Device Registration in Hong Kong: Essential Requirements and Procedures – Our Insights
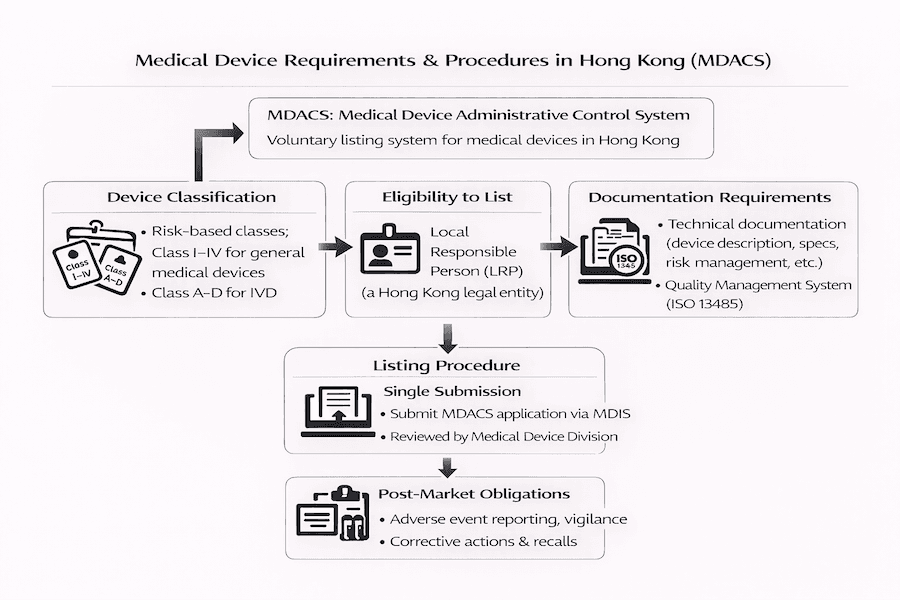
From what we've observed, getting your medical device listed in Hong Kong means navigating the Medical Device Administrative Control System (MDACS). This guide, built on our experience, aims to give you a clear, step-by-step look at the essential requirements and procedures involved. It’s all about making sure your device is ready for the Hong Kong healthcare market.
Essential Requirements: What Do You Absolutely Need?
So, what are the fundamental things you absolutely must have in place? Let's dive in:
1. Device Classification: How Risky Is Your Device?
Have you ever wondered how medical devices are categorized in Hong Kong? According to our understanding, MDACS uses a risk-based system, putting devices into four main classes:
- Class I (Low-risk devices): Think simple, everyday items like non-medicated bandages or crutches.
- Class II (Medium-risk devices): These include things like hypodermic needles or electronic thermometers.
- Class III (High-risk devices): More complex, such as lung ventilators or contact lenses.
- Class IV (Highest-risk devices): Critical items like heart valves or implantable defibrillators.
Why is this classification so important? Because, in our experience, the higher your device's risk class, the more stringent the regulatory checks will be, and the more extensive the evidence you'll need to provide for medical device registration Hong Kong.
2. Manufacturer Requirements: What's Expected of You?
As a manufacturer, you've got some key responsibilities. What do we typically see as crucial here?
- Must have a valid manufacturing license: This is your basic permit to operate.
- Must implement a quality management system: According to our understanding, this usually means having a system like ISO 13485 in place to ensure consistent quality. This is vital for medical device compliance Hong Kong.
- Must maintain technical documentation: Keep detailed records of your device's design, manufacturing, and testing.
- Must comply with international standards: Aligning with global benchmarks helps ensure your device meets high safety and performance criteria.
3. Local Responsible Person (LRP): Your Hong Kong Partner
For foreign manufacturers, appointing a Local Responsible Person (LRP) is non-negotiable. So, what exactly does an LRP do, and why are they so vital?
- Must be a Hong Kong registered company: They need to be a legal entity based locally.
- Must have a valid business registration: This proves their legitimacy.
- Must maintain proper documentation: They help keep all your local records in order.
- Must ensure regulatory compliance: In our experience, the LRP acts as your primary liaison with the Medical Device Division (MDD), making sure all Hong Kong medical device regulations are met. This role is essential for LRP Hong Kong requirements.
Registration Procedures: How Do You Get Listed?
Ready to get your device officially listed? Here's our step-by-step guide to the procedures:
1. Pre-submission Preparation: Getting Everything in Order
Before you hit "submit," thorough preparation is key to avoiding delays. What should you be focusing on at this stage?
- Complete application forms: Fill out all necessary forms accurately.
- Prepare technical documentation: This is where your Summary Technical Documentation (STED) comes into play, a concise summary of your device's technical file.
- Organize supporting documents: Gather all certificates, test reports, and other evidence.
- Ensure all requirements are met: Double-check everything against MDACS guidelines.
2. Documentation Requirements: What Paperwork Do You Need?
What kind of detailed information will the MDD be looking for? From our experience, a comprehensive application dossier usually includes:
- Device description and specifications: What is your device, and what are its key features?
- Manufacturing information: How is it made, and where?
- Quality control procedures: How do you ensure its quality during production?
- Risk analysis and management: What are the risks, and how are you controlling them?
- Clinical evaluation data: Evidence of your device's performance in real-world settings.
- Labeling and instructions: All packaging, device labels, and instructions for safe use. This is where additional labelling requirements Hong Kong (TR-005) come in, ensuring bilingual content.
3. Quality Management System: Proving Your Quality Commitment
A robust Quality Management System (QMS) is central to MDACS. What does this mean in practice for you?
- Must comply with ISO 13485: This is the internationally recognized standard for medical device QMS.
- Must include quality control procedures: Steps to monitor and maintain product quality.
- Must maintain proper documentation: Keep records of all QMS activities.
- Must conduct regular audits: Periodically review your QMS to ensure it's effective.
Special Considerations: Unique Situations
Are there any unique situations that require extra attention? Absolutely. Based on our experience, here are some key areas:
1. Custom-Made Devices: Built Just for One
What if your device is made specifically for a single patient? These "custom-made" devices have distinct rules:
- Special documentation requirements: More personalized records are often needed.
- Quality control measures: Ensuring quality even for one-off production.
- Risk management procedures: Assessing risks specific to the individual patient.
- Post-market surveillance: Even custom devices need monitoring.
2. Clinical Investigation: Testing in Real Life
If your device needs clinical trials, there are specific steps. What should you prepare for?
- Approval requirements: Getting the necessary green light for your study.
- Documentation needs: Detailed plans and records of the investigation.
- Safety monitoring: Constantly checking for any adverse events during the study.
- Reporting requirements: Submitting findings to the MDD.
3. Import/Export Requirements: Moving Your Device Across Borders
Thinking of bringing your device into Hong Kong or sending it out? You'll need to know about:
- Import license requirements: Does your device need a special permit to enter? This is relevant for medical device import Hong Kong.
- Export documentation: What paperwork is needed for international shipping?
- Customs clearance procedures: Navigating the logistics of border control.
- Regulatory compliance: Ensuring you meet all trade and safety regulations.
Post-Market Requirements: What Happens After Listing?
Is getting listed the end of your responsibilities? According to our experience, definitely not! Maintaining medical device compliance Hong Kong is an ongoing commitment.
1. Vigilance System: Keeping a Watchful Eye
How do you keep devices safe once they're on the market? Hong Kong's MDACS emphasizes a robust vigilance system:
- Adverse event reporting: Promptly telling the MDD about any problems or incidents. This is crucial for medical device adverse event reporting Hong Kong.
- Field safety corrective actions: Taking necessary steps to fix issues with devices already distributed.
- Recall procedures: Having a clear plan for withdrawing unsafe devices from the market.
- Communication requirements: Keeping all stakeholders informed.
2. Ongoing Compliance: The Continuous Journey
What does continuous compliance mean in practice?
- Documentation updates: Keeping your technical files and records current.
- Quality system maintenance: Regularly reviewing and improving your QMS.
- Regular audits: Conducting internal and external checks to ensure adherence to standards.
- Change management: Properly managing and reporting any modifications to your listed device (as per GN-10). This ensures continuous post-market surveillance Hong Kong.
Contact Information
For further information or assistance, we always recommend reaching out to the official source:
- Medical Device Division, Department of Health
- Website: www.mdd.gov.hk
Important Notes: Our Practical Advice
Based on our experience, here are some key takeaways to keep in mind:
1. Compliance Timeline: Plan Ahead!
- Allow sufficient time for review: Registration processes can take a while, so patience and planning are key.
- Plan for additional information requests: It's common for the MDD to ask for more details; responding quickly is vital.
- Consider post-market requirements: Factor in ongoing vigilance and compliance costs from the start.
- Monitor regulatory updates: Stay informed about any changes to the rules.
2. Best Practices: Our Top Tips for Success
- Early engagement with MDD: Don't hesitate to reach out with questions.
- Complete documentation preparation: A thorough dossier speeds up the process significantly.
- Regular compliance monitoring: Keep an eye on your internal processes.
- Maintain proper records: Good record-keeping makes everything easier in the long run.
3. Cost Considerations: What About the Investment?
What kind of costs should you anticipate?
- Application fees: Fees for submitting your listing application.
- Testing costs: Costs associated with required safety and performance tests.
- Ongoing compliance costs: Expenses for maintaining your QMS, LRP services, and post-market activities.
- Documentation maintenance: The cost of keeping your technical files updated.
To our understanding, by keeping these aspects in mind, you can approach medical device registration in Hong Kong with confidence and ensure a smoother pathway to market success.
Ask Anything
We'll follow up with you personally.
Related Articles
Approximately 5 minutes
Medical Device Adverse Event Reporting in Hong Kong: A Guide for LRPs
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Medical Device Regulations and Registration in Hong Kong
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Navigating Medical Device Regulations in Hong Kong: The MDACS Framework
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.
Approximately 5 minutes
Listing Procedures for Local Medical Device Manufacturers in Hong Kong: A Guide to GN-08
This article details the application process for local medical device manufacturers seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-08. It covers eligibility, quality management system requirements, and the submission process, all based on our insights and experience for effective Hong Kong medical device manufacturing.
Approximately 5 minutes
Listing Procedures for Medical Device Importers in Hong Kong: A Guide to GN-07
For entities importing medical devices into Hong Kong, the Medical Device Administrative Control System (MDACS) provides a voluntary listing scheme for importers, guided by GN-07. This article details the eligibility, application steps, and key requirements for listing as a medical device importer, based on our insights and experience for efficient Hong Kong medical device import.
Approximately 5 minutes
Listing Procedures for Medical Device Distributors in Hong Kong: A Guide to GN-09
This article outlines the application process for medical device distributors seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as detailed in GN-09. It covers eligibility, key requirements for documented procedures, and the submission process to enhance traceability and public safety, based on our insights.
Approximately 5 minutes
Listing Procedures for Class B, C, and D In Vitro Diagnostic Medical Devices in Hong Kong: A Guide to GN-06
This article details the application process for listing Class B, C, and D In Vitro Diagnostic Medical Devices (IVDMDs) under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-06. It covers classification, eligibility, submission requirements, and the online application via MDIS, all based on our insights and experience for efficient IVDMD listing in Hong Kong.